Dinesh Chandra Doval1, Saud Azam2, Ullas Batra3, Kumardeep Dutta Choudhury3, Vineet Talwar3, Sunil Kumar Gupta3, Anurag Mehta4
1 Department of Medical Oncology; Department of Research, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India
2 Department of Research, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India
3 Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India
4 Department of Laboratory Services, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India
| Date of Submission | 16-Apr-2013 |
| Date of Acceptance | 12-May-2013 |
| Date of Web Publication | 12-Jul-2013 |
Correspondence Address:
Dinesh Chandra Doval
Department of Medical Oncology; Department of Research, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1477-3163.114970
Abstract
Background: Adenocarcinoma, a subgroup of non-small cell lung cancer, is the most frequent form occurring in the non-smokers. Mutation in tyrosine kinase domain of epidermal growth factor receptor (EGFR) has been a common feature observed in lung adenocarcinoma. The study was carried out to detect the prevalence of EGFR mutation in lung adenocarcinoma. Materials and Methods: EGFR mutation status in 166 lung adenocarcinoma patients was obtained retrospectively. Mutation tests were performed on paraffin embedded tissue blocks as a routine diagnostic procedure by polymerase chain reaction followed by direct nucleotide sequencing. Patient’s demographics and other clinical details were obtained from the medical records. Results: EGFR mutation was detected in 43/166 (25.9%) patients. Gender wise mutation was observed as 18/55 (32.7%) in females and 25/111 (22.5%) in males. Overall, EGFR mutation was correlated with never smokers and distant metastasis ( P < 0.05), but not associated with the gender, disease stage and pleural effusion. Exon 19 deletions were significantly correlated with females, never smokers, pleural effusion and distant metastasis ( P < 0.05). However, point mutation on exon 21 did not show any statistical association with the above variables. Median overall survival was 22 months (95% confidence interval, 15.4-28.6). Female sex, EGFR mutation and absence of metastasis are associated with good prognosis. Conclusion: EGFR mutation in lung adenocarcinoma was higher in never smokers, females and patients with distant metastasis. However, it was not linked with tobacco smoking. The prevalence of EGFR mutation observed is in range with the previously published reports from the Asian countries.
Keywords: Adenocarcinoma, epidermal growth factor receptor, mutation, non-small cell lung cancer
How to cite this article:
Doval DC, Azam S, Batra U, Choudhury KD, Talwar V, Gupta SK, Mehta A. Epidermal growth factor receptor mutation in lung adenocarcinoma in India: A single center study. J Carcinog 2013;12:1
How to cite this URL:
Doval DC, Azam S, Batra U, Choudhury KD, Talwar V, Gupta SK, Mehta A. Epidermal growth factor receptor mutation in lung adenocarcinoma in India: A single center study. J Carcinog [serial online] 2013 [cited 2021 Oct 13];12:12. Available from: https://carcinogenesis.com/text.asp?2013/12/1/12/114970
Introduction
Lung cancer is a commonly diagnosed malignancy and among one of the leading causes of death around the world as well as in India. It accounts for 13% (1.6 million) of the total cancer cases and 18% (1.4 million) of the total cancer deaths world-wide in the year 2008. [1] It is most frequently diagnosed malignancy in Indian males and the 4 th most common together in both the sexes. [2] In Delhi, lung cancer is the commonest malignancy in males and the 6 th in females. In terms of cancer deaths, it acquires the highest position in males and the 5 th position in females. [3] Lung cancer is usually associated with positive smoking history. However, of all the lung cancers diagnosed in the world, approximately 25% were never smokers. [4] Non-small cell lung cancer (NSCLC) data in non-smokers from India were higher and reported from 52.7% to 39.5%. [5],[6] Adenocarcinoma, a subgroup of NSCLC, is the most common form occurring in the non-smokers and smokers as well. [7] It generally presents in an advanced stage of the disease leaving a limited treatment option. Response to the standard chemotherapy regimens is poor with short median survival.
Epidermal growth factor receptor (EGFR) has been associated with tumorigenesis in several malignancies including NSCLC. [8],[9] Studies have also reported overexpression with the reduced survival in lung cancer. [8] EGFR is a transmembrane receptor protein kinase and is a member of the avian erythroblastic leukemia viral oncogene homolog (ErbB) family of receptors, which includes EGFR (ErbB-1), HER2/neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4). [10] Receptor binding to the extracellular domain of EGFR causes protein kinase activation in the form of downstream signaling that lead to the growth, proliferation and invasion of the cell. [11] Somatic mutation in the tyrosine kinase (TK) domain of EGFR has been a common feature observed in lung adenocarcinoma, [12] which is being exploited in lung cancer treatment with TK inhibitors such as gefitinib and erlotinib, [13],[14] that blocks the cell signaling by binding to the TK domain. More recently, afatinib (a new molecule which is an irreversible ErbB family blocker) has shown promising results in a phase IIb/III trial by increasing the progression free survival in lung cancer patients who progressed after systemic chemotherapy and gefitinib/erlotinib therapy. [15]
Mutations in the EGFR gene are generally associated with adenocarcinoma histology of lung cancer, female sex, non-smokers and Asian ethnicity. [16],[17] EGFR mutation data are available mostly from the population of East Asia ranging from 24% to 66.3%. [18],[19],[20] Studies from USA and Europe reported low mutation rates (13-17%) in these populations. [12],[21] A few reports are available from India as well which showed the overall EGFR mutation in lung adenocarcinoma as 44-55%. [22],[23] Exons 19-21 are the most commonly studied exons, which generally serve as mutation hotspots. In-frame deletions and point mutations are the most frequently found mutations. [10],[12],[18],[22] There are variations in the frequency of mutation observed within India. [23] More information and data from India is warranted to establish the rate of mutation in this ethnic group/Indian subcontinent. The present study was carried out to detect the prevalence of EGFR mutation in lung adenocarcinoma in a tertiary care hospital in India.
Materials and Methods
One hundred-sixty six patients of lung adenocarcinoma with known EGFR mutation status (in routine diagnostic) were sequentially included in this study. Patient’s demographics, history and treatment details were obtained from the medical records. The data and information were collected retrospectively from a period of November 2009 to April 2012.
Paraffin embedded tissue blocks (biopsies or surgically resected specimens), from primary tumors as well as from metastatic sites, were used for the mutation analysis. Genomic deoxyribonucleic acid was extracted and exons 18-21 of EGFR gene were amplified by polymerase chain reaction (PCR). The amplified PCR product was subjected to the direct nucleotide sequencing for the detection of mutations. Primers for the PCR and methods were used as described previously. [13]
Statistical analysis was performed using Pearson Chi-square or Fisher’s exact test, whichever was appropriate for categorical variables. Kaplan-Meier method was applied for survival estimate and log rank test for comparing the difference between the subgroups. Cox proportional hazard method was used for the multivariate analysis. A two sided P < 0.05 was considered significant. Statistical Package for the Social Sciences (SPSS) version 16 for Windows (SPSS Inc. Chicago, IL, USA) was used in performing all the statistical analysis.
Institutional Review Board of Rajiv Gandhi Cancer Institute and Research Centre had granted waiver for this study as the mutation analysis of EGFR is a part of the routine clinical practice that helps in decision making and treatment planning of the patient. Information and the detailed of history of patients were collected in a de-identified manner.
Results
We included 166 NSCLC adenocarcinoma patients in this study, of which 111 (69.9%) were males [Table 1]. Median age of patients was 60 ± 9.36 (range 34-79) years. Among all patients, 57.2% were never smokers. EGFR mutation was detected in 43/166 (25.9%) patients. High mutation frequency was observed among the females (32.7%) than in males. Of all the mutations, the most predominant were in-frame deletion on exon 19 (51.2%) followed by point mutation on exon 21 (34.9%). The in-frame deletions comprises of del746-750 [Figure 1] and del747-752 and point mutation on exon 21 of L858R (14 cases) and L861Q (1 case). Point mutation G719A was observed in only one case on exon 18. Two insertions – ins774A and ins775H and D770-N771 ins SVD were seen in the exon 20 of EGFR gene. There were three cases where mutation on two exons were detected simultaneously, these were exons 20, 21 (T790M, L858R); exons 19, 21 (D761Y and L858R); exon 19, 20 (del746-750, Q787R).
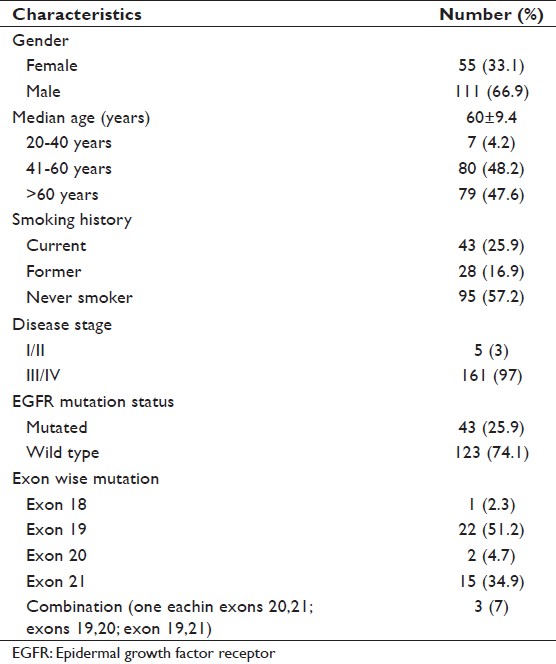 |
Table 1: Patient demographics (N=166) Click here to view |
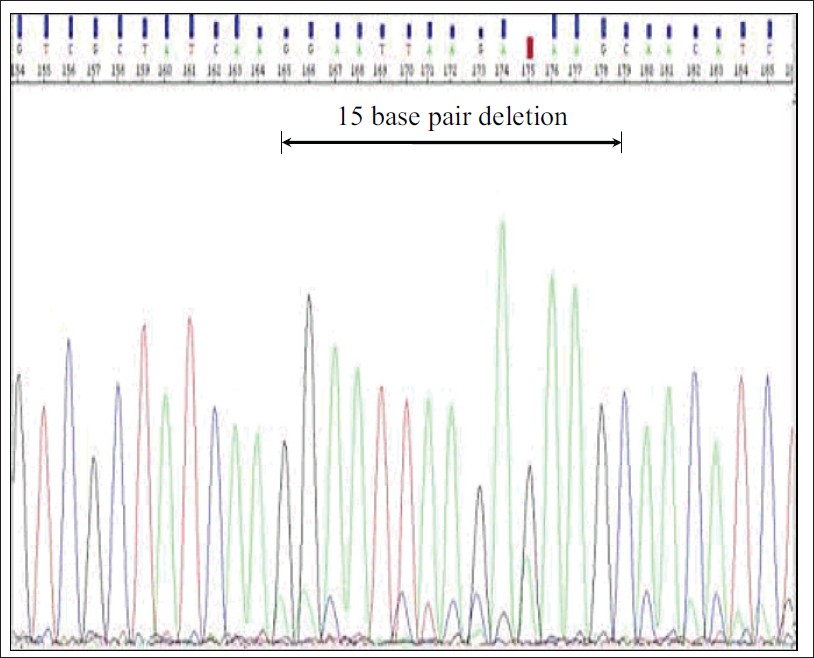 |
Figure 1: Deletion of 15 base pairs on exon 19 Click here to view |
A total 74% of patients presented with metastatic disease and the common sites of metastasis were bone (42.6%), brain (22.8%), liver (13.8%) and adrenal gland (9.6%). EGFR mutation was associated with never smokers and distant metastasis (P < 0.05), but it did not significantly correlate with the gender, disease stage and pleural effusion [Table 2]. However, a near significant correlation was observed in the patients in the age group 41-60 years (P = 0.054). The above mentioned variables were also compared with mutations in exon 19 and exon 21 (most predominant mutations in the study). Exon 19 deletions were significantly correlated with female gender, never smokers, pleural effusion and distant metastasis (P < 0.05). Point mutation on exon 21 was predominant in males; however, the result was not statistically significant [Table 3].
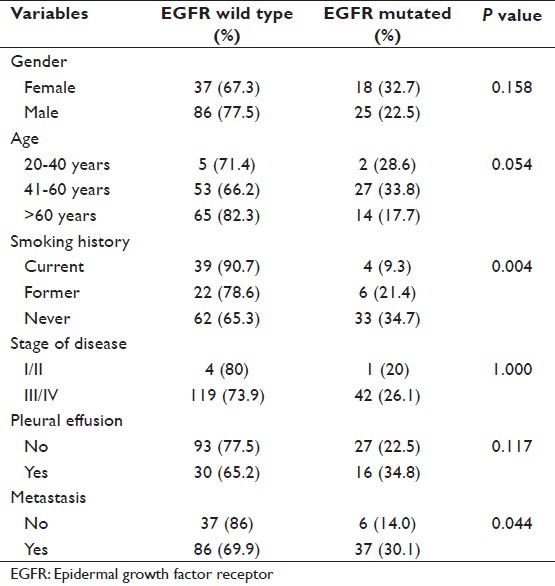 |
Table 2: Frequency of overall EGFR mutation and its correlation (N=166) Click here to view |
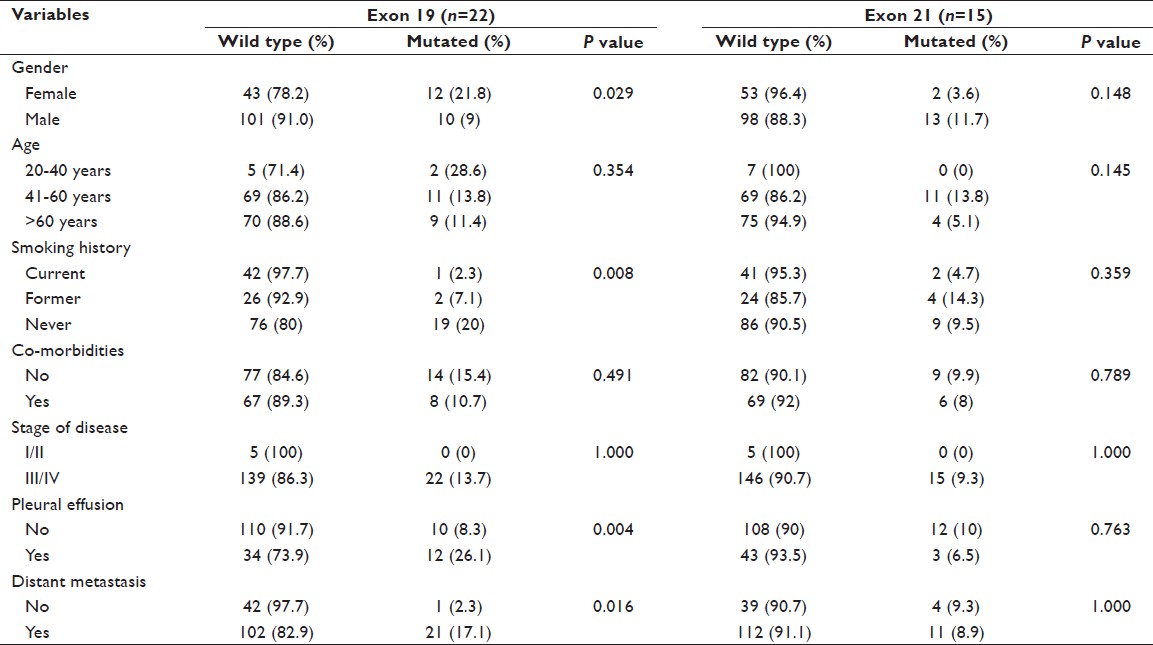 |
Table 3: Exon-wise distribution of mutation and its correlation (N=166) Click here to view |
At the time of analysis, the survival status of 153/166 patients were known and hence included in the survival analysis. Mean follow-up was 12.3 months. Fifty eight out of 153 patients were deceased. Median overall survival (OS) was 22 months (95% confidence interval [CI], 15.4-28.6) [Figure 2]. Median OS was 16 months (95% CI, 11.6-20.4) in EGFR wild type and 31 months (95% CI, 17.1-44.9) in EFGR mutant patient (P = 0.024) [Figure 3]a. When gender was taken into account the median OS was 31 months in females (95% CI, 22.3-39.7) than 14 month (95% CI, 9.8-18.2) in males and the result was a near significant (P = 0.051) [Figure 3]b. Median survival was 23 months (95% CI, 13.2-32.8) for the age group 20-40 years, 13 months (95% CI, 17.8-44.2) for age group 41-60 years and 14 months (95% CI, 6.9-21.0) in age group more than 60 years (P = 0.032) [Figure 3]c. Distant metastasis was also a significant factor that effect survival. Median OS was 44 months in the patient presented without metastasis (95% CI, 12.6-75.2) and 17 months (95% CI, 10.9-23.1) in patients presented with metastasis at distant a site (P = 0.009) [Figure 3]d. Current smoker population showed shorter median OS (12 months) than never and former smoker population (27 and 22 months), however, the result was not statistically significant on log rank test. Other factors like presence or absence of co-morbidities (23 months vs. 16 months) and presence or absence pleural effusion (13 months vs. 23 months) also not achieved a statistical significance. Multivariate analysis using Cox proportional hazard have shown EGFR mutation (hazard ratio, 0.411; 95% CI, 0.189-0.894; P = 0.025), female gender (hazard ratio, 2.227; 95% CI, 1.173-4.226; P = 0.014) and absence of metastasis (hazard ratio, 3.248; 95% CI, 1.577-6.689; P = 0.001) to be associated with longer median OS, thereby indicating a good prognostic factor.
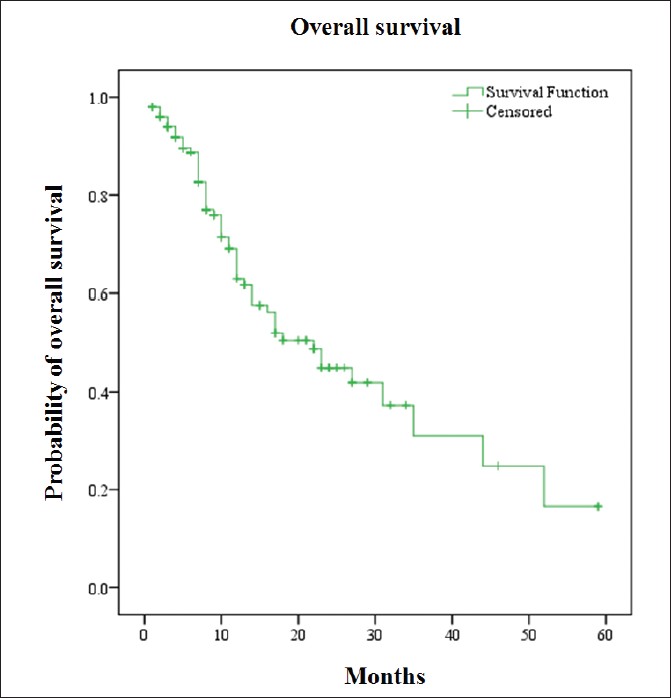 |
Figure 2: Kaplan-Meier curve for overall survival Click here to view |
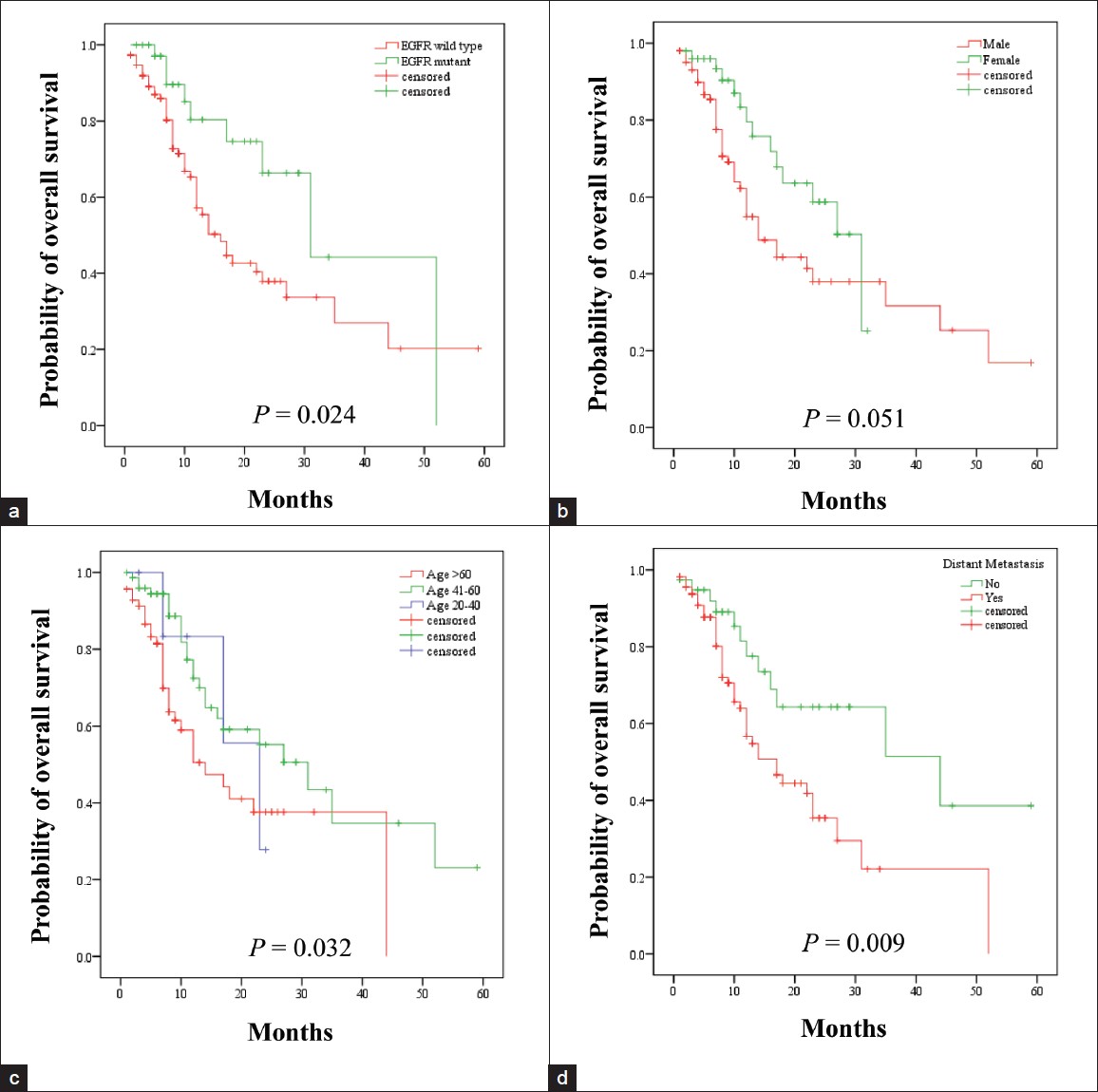 |
Figure 3: Kaplan-Meier curve for overall survival according to (a) epidermal growth factor receptor mutation, (b) sex, (c) age and (d) distant metastasis at the time of presentation Click here to view |
Chemotherapy alone or in combination with radiotherapy (RT) and oral targeted therapy was the foremost treatment modality adopted to treat these patients. Common sites of RT were brain (48.6%), bone (31.4%) and lung (17.1%). Pemetrexed combined with platinum compounds (carboplatin or cisplatin) in a palliative setting was the main chemotherapy regimen while whole brain RT was generally offered to the patients presenting with symptomatic brain metastasis. Ninety percent of the patients who were EGFR mutation positive received oral targeted therapy in the form of erlotinib or gefitinib.
Discussion
NSCLC is the largest subgroup of lung cancer harboring a majority of activating mutations in kirsten rat sarcoma viral oncogene homolog (K-ras) codon 12 and 13, p53 and EGFR. [24] Recently discovered echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) rearrangement by Soda et al., is sensitive to the ALK inhibitors. [25] Targeting these mutations has changed the overall treatment strategy for lung cancer and provides significant benefit to the patient. EGFR mutation screening is becoming a standard of care in the oncology clinical practice and treatment with inhibitors showed efficacy and increase in the survival of patients with EGFR mutation positive lung cancer. In this paper, we reported the prevalence of EGFR mutation in lung cancer in India with the data from a single institute.
Lung cancer adenocarcinoma possesses higher EGFR mutation rates than any other form of NSCLC. [14],[26] In the present study, we examined mutation in the TK domain of EGFR which is most common factor of the activation of this receptor on the cell surface. [13],[27] Overall mutation in the present study was 25.9% which falls in the range of 10-66%, [20],[28] reported in the published papers, irrespective of region and ethnicity. Studies from Korea, [18] Taiwan, [29] Japan, [30] and China, [7] have observed EGFR mutation rates as 24%, 50.5%, 26.3% and 38.1%, respectively. In the populations from Europe and America, frequency of EGFR mutation in lung adenocarcinoma has been observed as 17.3%, [12] 10.6%, [28] and 19%. [31] Our result is in line with reports from Asia. However, a study from India by Sahoo et al., has reported mutation frequency in lung adenocarcinoma as 44%. [22] Regional difference has also been reported within India as 65% mutation observed in southern Indian population and 33% in northern population, according to an abstract presented in a conference by Aggarwal et al. [23] Our study population comprises of northern part of India showing a mutation rate as 25.9%. The variation in the frequency observed around the world may be because of environmental, geographical and ethnic differences, different methodologies of mutation detection and exclusion of less occurring mutation like exon 18 in some studies. [28],[32] If there is a notable difference within India, it needs further evaluation by prospective population based studies. It could be suggested that there may be a presence of some unknown carcinogens in the atmosphere of this part of world that could lead to the mutation in this gene; however, this hypothesis needs to be explored.
Point mutation on exon 21 and in-frame deletions on exon 19 of EGFR is the most commonly observed mutation and accounts for 80-90% of all the mutations detected. [33],[34] Our data shows a cumulative mutation of these two exons as 86.1%, which confirms the published reports that have pointed the dominance of mutation in these two exons. Dual mutations in the EGFR gene have also been observed in our study and these generally occur in combination with exon 19 deletion or exon 21 point mutation which provides us a hint that these mutations generally get activated in combination with the frequently occurring mutation.
Higher EGFR mutation rate was observed in females than males (32.7% vs. 22.5%, P = 0.158) in the present study, but the result was not statistically significant. However, this observation is consistent with the previously published studies that have shown the female gender dominance in the EGFR mutation. [7],[28],[35] Exon-wise comparison of mutations provide us a positive correlation of female sex, never smokers, presence of metastasis and pleural effusion with exon 19 deletions ( P < 0.05). However, exon 21 did not show any correlation with these variables. A similar result was reported from a study, [22] that showed female sex and never smoking to be associated with exon 19 deletions, but not associated with exon 21 point mutation. On the other hand, Tanaka et al., [35] reported the male gender to be associated with exon 19 deletions. The reason for female sex bias is not well-understood; however, Gazdar and Thun, [36] reported that estrogen and its receptors probably influence polymorphic genes expression that regulates tobacco carcinogen metabolism thereby promoting the lung carcinogenesis.
Age has been categorized in three groups in this study and high mutation rate (62.8%) was observed in the age group of 41-60 years ( P = 0.054), which is nearly significant as per the statistics. A similar result has also been observed ( P = 0.06) with the age group 40-60 years, previously. [22] It could be suggested that the risk of mutation increases with increase in age that lead to the development of lung cancer. Tobacco smoking is a well-established factor and one of the major causes in lung cancer development. Lung cancer in never smokers is also frequently experienced world-wide. Our data of NSCLC in never smoker population (54.7%) is slightly higher than those of previously published reports from India by Krishnamurthy et al. (52.7%), [5] and Malik et al. (39.5%), [6] and is also higher in females than males. There has been an increasing trend of diagnosis of NSCLC with adenocarcinoma histology in never smoker group, world-wide and in India as well. In the present study, a positive correlation of EGFR mutation was established with the never smokers than the current and former smokers (54.7%, 21.4%, 9.3%; P = 0.004). The data confirms the previous studies which have also established a relationship of EGFR mutation with non-smokers. [14],[26],[37] We observed that 96.4% females and 37.8% males were never smokers in our study while there was much lower percentage of never smokers in the western population. The difference in the smoking habits is reflected in the EGFR mutation data from around the world. Takano et al., [32] suggested that EGFR mutation occurs in a random manner in the population, regardless of the smoking habits and other genetic mutations like K-ras, which occurs more frequently and EGFR less frequently in the smoker population. A review by Lee et al., [24] have also showed EGFR mutation in the never smokers as a single genetic event in lung cancer rather than other genetic changes like K-ras, p53 which are more commonly observed in the smoker population. Another study indicated that carcinogens in the tobacco smoke mutate the Ras gene, however, in the never smokers where carcinogen is not present, the upstream pathway of EGFR gene is activated by unknown factors. [36] These findings indicate that lung cancer in EGFR positive cases does not occur via the carcinogenic process and pathways induced by tobacco and its product, rather it follows some other unknown pathways of carcinogenesis. These processes lead to the uncontrolled proliferation and invasion of cancer cell, which may develop metastasis at a distant site.
In NSCLC, the majority of patients present with an advanced stage of the disease. Correlation of EGFR mutation and disease stage was not statistically significant even though majority of mutations were detected in patients with advanced stage (III/IV). However, EGFR mutation in the patients with distant metastasis was correlated statistically ( P = 0.044). Frequently observed distant metastatic sites in this study were bone (43%), brain (22.2%), liver (14.1%) and adrenal gland (9.6%). Togashi et al. [38] correlated the presence of pulmonary metastasis with the EGFR mutation ( P < 0.05), but their data is limited only to a particular metastatic site. In another study, mutation was associated with brain metastasis. [10] A recently published study in Dutch population, [28] and another study from Asia, [29] reported an increased EGFR mutation frequency in patients with pleural effusion. It may be suggested that EGFR mutation activates some unknown pathway involved in the process of metastasis. It may also be suggested that patients who have metastasis and pleural effusion in whom interventional biopsies are not possible may be considered as candidates for TK inhibitor therapy. [29]
The survival analysis has revealed that female sex, presence of EGFR mutation and absence of distant metastasis are associated with improved median OS and also indicated as a good prognostic factor in a multivariate analysis. Previous studies have also reported a similar trend with female sex and EGFR mutation as indicators of good survival. [12],[18],[29] The higher median OS in patients with EGFR mutation could be due to the better response to systemic chemotherapy and EGFR TK inhibitors.
The present study has limitations due to its retrospective nature and some selection bias in terms of patients whose EGFR mutation was known or patients with advanced stage of the disease.
Conclusion
The prevalence of EGFR mutation observed is in range with the previously published reports from the Asian countries. It is not associated with tobacco smoking as shown in our study as well as previously published reports, which indicates the involvement of an alternative mechanism of carcinogenesis other than tobacco and its products. Further, EGFR mutation in lung adenocarcinoma is linked with females of Asian ethnicity, never smokers and in patients presenting with distant metastasis. Being responsive to oral TK inhibitors, the screening of this somatic mutation especially in females and/or never smokers could be very helpful in the further disease management of these patients.
Acknowledgment
We acknowledge Rupal Sinha for her helpful discussion.
References
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.  [PUBMED] |
| 2. | International Agency for Research on Cancer. GLOBOCAN, 2008. Available from: http://globocan.iarc.fr/. [Last accessed on 2012 Dec 8].  |
| 3. | National Cancer Registry Programme, Indian Council of Medical Research. Three year report of population based cancer registries (2006-2008) 2010.  |
| 4. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917.  [PUBMED] |
| 5. | Krishnamurthy A, Vijayalakshmi R, Gadigi V, Ranganathan R, Sagar TG. The relevance of “Nonsmoking-associated lung cancer” in India: A single-centre experience. Indian J Cancer 2012;49:82-8.  [PUBMED] |
| 6. | Malik PS, Sharma MC, Mohanti BK, Shukla NK, Deo S, Mohan A, et al. Clinico-pathological profile of lung cancer at AIIMS: A changing paradigm in India. Asian Pac J Cancer Prev 2013;14:489-94.  [PUBMED] |
| 7. | Huang YS, Yang JJ, Zhang XC, Yang XN, Huang YJ, Xu CR, et al. Impact of smoking status and pathologic type on epidermal growth factor receptor mutations in lung cancer. Chin Med J (Engl) 2011;124:2457-60.  |
| 8. | Inamura K, Ninomiya H, Ishikawa Y, Matsubara O. Is the epidermal growth factor receptor status in lung cancers reflected in clinicopathologic features? Arch Pathol Lab Med 2010;134:66-72.  [PUBMED] |
| 9. | Ohsaki Y, Tanno S, Fujita Y, Toyoshima E, Fujiuchi S, Nishigaki Y, et al. Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol Rep 2000;7:603-7.  |
| 10. | Matsumoto S, Takahashi K, Iwakawa R, Matsuno Y, Nakanishi Y, Kohno T, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer 2006;119:1491-4.  [PUBMED] |
| 11. | Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp Cell Res 2003;284:31-53.  [PUBMED] |
| 12. | Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67.  [PUBMED] |
| 13. | Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39.  |
| 14. | Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11.  [PUBMED] |
| 15. | Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38.  [PUBMED] |
| 16. | Ma BB, Hui EP, Mok TS. Population-based differences in treatment outcome following anticancer drug therapies. Lancet Oncol 2010;11:75-84.  [PUBMED] |
| 17. | Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol 2006;11:190-8.  [PUBMED] |
| 18. | Jang TW, Oak CH, Chang HK, Suo SJ, Jung MH. EGFR and KRAS mutations in patients with adenocarcinoma of the lung. Korean J Intern Med 2009;24:48-54.  [PUBMED] |
| 19. | Bae NC, Chae MH, Lee MH, Kim KM, Lee EB, Kim CH, et al. EGFR, ERBB2, and KRAS mutations in Korean non-small cell lung cancer patients. Cancer Genet Cytogenet 2007;173:107-13.  [PUBMED] |
| 20. | Gao B, Sun Y, Zhang J, Ren Y, Fang R, Han X, et al. Spectrum of LKB1, EGFR, and KRAS mutations in chinese lung adenocarcinomas. J Thorac Oncol 2010;5:1130-5.  [PUBMED] |
| 21. | Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 2005;23:5900-9.  [PUBMED] |
| 22. | Sahoo R, Harini VV, Babu VC, Patil Okaly GV, Rao S, Nargund A, et al. Screening for EGFR mutations in lung cancer, a report from India. Lung Cancer 2011;73:316-9.  [PUBMED] |
| 23. | Aggarwal S, Patil S, Minhans S, Pungliya M, Soumitra N. A study of EGFR mutation in nonsmoker NSCLC: Striking disparity between north and south India patients [abstract]. J Clin Oncol 2012;30 Suppl:E18041.  |
| 24. | Lee YJ, Kim JH, Kim SK, Ha SJ, Mok TS, Mitsudomi T, et al. Lung cancer in never smokers: Change of a mindset in the molecular era. Lung Cancer 2011;72:9-15.  [PUBMED] |
| 25. | Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6.  [PUBMED] |
| 26. | Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46.  [PUBMED] |
| 27. | Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500.  |
| 28. | Smits AJ, Kummer JA, Hinrichs JW, Herder GJ, Scheidel-Jacobse KC, Jiwa NM, et al. EGFR and KRAS mutations in lung carcinomas in the Dutch population: Increased EGFR mutation frequency in malignant pleural effusion of lung adenocarcinoma. Cell Oncol (Dordr) 2012;35:189-96.  [PUBMED] |
| 29. | Wu SG, Gow CH, Yu CJ, Chang YL, Yang CH, Hsu YC, et al. Frequent epidermal growth factor receptor gene mutations in malignant pleural effusion of lung adenocarcinoma. Eur Respir J 2008;32:924-30.  [PUBMED] |
| 30. | Usui K, Ushijima T, Tanaka Y, Tanai C, Noda H, Abe N, et al. The frequency of epidermal growth factor receptor mutation of nonsmall cell lung cancer according to the underlying pulmonary diseases. Pulm Med 2011;2011:290132.  [PUBMED] |
| 31. | Reinersman JM, Johnson ML, Riely GJ, Chitale DA, Nicastri AD, Soff GA, et al. Frequency of EGFR and KRAS mutations in lung adenocarcinomas in African Americans. J Thorac Oncol 2011;6:28-31.  |
| 32. | Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol 2005;23:6829-37.  [PUBMED] |
| 33. | Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res 2006;12:7232-41.  [PUBMED] |
| 34. | Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81.  [PUBMED] |
| 35. | Tanaka T, Matsuoka M, Sutani A, Gemma A, Maemondo M, Inoue A, et al. Frequency of and variables associated with the EGFR mutation and its subtypes. Int J Cancer 2010;126:651-5.  [PUBMED] |
| 36. | Gazdar AF, Thun MJ. Lung cancer, smoke exposure, and sex. J Clin Oncol 2007;25:469-71.  [PUBMED] |
| 37. | Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, et al. EGFR mutations in non-small-cell lung cancer: Analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005;23:857-65.  [PUBMED] |
| 38. | Togashi Y, Masago K, Kubo T, Sakamori Y, Kim YH, Hatachi Y, et al. Association of diffuse, random pulmonary metastases, including miliary metastases, with epidermal growth factor receptor mutations in lung adenocarcinoma. Cancer 2011;117:819-25.  [PUBMED] |
Authors
Dr. Dinesh Chandra Doval: Department of Medical Oncology and Department of Research, Rajiv Gandhi Cancer Institute and Research Centre, Sector – 5, Rohini, Delhi – 110 085, India.
Mr. Saud Azam: Department of Research, Rajiv Gandhi Cancer Institute and Research Centre, Sector – 5, Rohini, Delhi – 110 085, India.
Dr. Ullas Batra: Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Sector – 5, Rohini, Delhi – 110 085, India.
Dr. Kumardeep Dutta Choudhury: Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Sector – 5, Rohini, Delhi – 110 085, India.
Dr. Vineet Talwar: Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Sector – 5, Rohini, Delhi – 110 085, India.
Dr. Sunil Kumar Gupta: Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Sector – 5, Rohini, Delhi – 110 085, India.
Dr. Anurag Mehta: Department of Laboratory Services, Rajiv Gandhi Cancer Institute and Research Centre, Sector – 5, Rohini, Delhi – 110 085, India.
