Mariko Tomita
Department of Pathology and Oncology, Graduate School of Medical Science, University of the Ryukyus, Okinawa- 903-0215, Japan
| Date of Submission | 15-Dec-2011 |
| Date of Acceptance | 04-Mar-2012 |
| Date of Web Publication | 28-Jul-2012 |
Correspondence Address:
Mariko Tomita
Department of Pathology and Oncology, Graduate School of Medical Science, University of the Ryukyus, Okinawa- 903-0215
Japan
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1477-3163.99176
Abstract
Background: Caloric restriction (CR), a lowering of caloric intake without malnutrition, is associated with longevity. CR also decreases incidences of age-related diseases including cancer. The sirtuins (SIRTs) have been implicated as a key mediator for the beneficial effects of CR on longevity. However, the underlying mechanisms by which CR decreases cancer risk have not yet been fully elucidated. Materials and Methods: The present study was conducted to determine whether CR would modify the growth of preneoplastic colonic aberrant crypt foci (ACF). We also analyzed the expression of SIRTs to elucidate the molecular mechanisms of cancer-preventive effects of CR. F344 rats were fed a CR diet (60% of ad libitum diet) or a basal diet ad libitum. Then, the animals were given subcutaneous injection of either 1, 2-dimethylhydrazine (DMH) that enhances cell proliferation of colonic mucosa or saline. All animals were sacrificed at 5 weeks after the beginning of the experiment. Results: The number of ACF in colonic mucosa was significantly decreased in DMH-treated rats with CR as compared to in those without CR. No ACF was found in DMH-untreated animals with or without CR. Also, we found that CR decreased the cell proliferation of colonic mucosa in DMH-treated rats. The expressions of anti-apoptotic gene, Survivin, and cell cycle progression-associated gene, Cyclin D1, were increased by DMH-treatment. Both of the genes expressions were declined by CR in those of DMH-treated rats. The expressions of all SIRT1-7 mRNAs were significantly increased by CR in DMH-treated rats. Conclusion: As previous studies demonstrated that SIRT1 down-regulates Survivin and Cyclin D1, our findings suggest that at least SIRT1 protect colonic mucosa from formation and development of ACF by increasing apoptosis and reducing excessive cell growth in colon epithelial cells.
Keywords: 1, 2-dimethylhydrazine, aberrant crypt foci, caloric restriction, sirtuin
How to cite this article:
Tomita M. Caloric restriction reduced 1, 2-dimethylhydrazine-induced aberrant crypt foci and induces the expression of Sirtuins in colonic mucosa of F344 rats. J Carcinog 2012;11:10
How to cite this URL:
Tomita M. Caloric restriction reduced 1, 2-dimethylhydrazine-induced aberrant crypt foci and induces the expression of Sirtuins in colonic mucosa of F344 rats. J Carcinog [serial online] 2012 [cited 2021 Oct 14];11:10. Available from: https://carcinogenesis.com/text.asp?2012/11/1/10/99176
Background
Colorectal cancer is one of the major causes of morbidity and mortality worldwide. [1] Lifestyle and environmental factors affect cancer initiation, promotion, and progression, suggesting that many common cancers are preventable. Epidemiological [2],[3] and experimental [4] investigations have revealed the importance of dietary factors in the causation of colon cancer. Therefore, there is extensive interest in the relationship between the caloric intake and colon carcinogenesis. Caloric restriction (CR), defined as a 20-40% reduction in energy intake without malnutrition, extends lifespan and delays the onset and slows the progression of age-associated diseases such as diabetes mellitus, cardiovascular diseases, and cancer in several species. [5] The first scientific report, which described that CR inhibits the growth of tumors transplanted into mice, was published in early 20 century. There has been a revival of interest in the relationship between caloric intake and carcinogenesis. Previous studies have indicated that CR is associated with decreased incidence of spontaneous or chemically induced tumors in various animal tissues, including colon tumors. [6],[7] 1, 2-dimethylhydrazine (DMH)-induced colon tumors could be inhibited by 40% CR in rats. [7] In other study, azoxymethane (AOM)-induced rat colon tumors was significantly inhibited by restricting the calories by 30%. [8] Same group demonstrated that CR by about 10%, 20%, and 30% inhibited colon tumor incidence in a dose-dependent manner. They also demonstrated that ornithine decarboxylase and protein tyrosine kinase activities were lower in the colon tumors of animals fed the CR diets. [9] However, the molecular mechanisms underlying the protection of colon carcinogenesis by CR remain unclear.
Silent information regulator 2 (Sir2), which promotes longevity in yeast, C. elegans, and, Drosophila, is a nicotinamide adenosine dinucleotide (NAD+)-dependent class III histone deacetylase (HDAC). [10] The expression or activity of Sir2 is increased by CR. Therefore, Sir2 in lower organisms play a critical role in promoting longevity. [11] In mammals, there are seven SIRTs, SIRT1-7, which are localized to the nucleus, cytoplasm, or mitochondria depending on the different orthologous. They target a wide range of cellular proteins for post-translational modification by deacetylation (SIRT1, 2, 3, and 5) or ADP ribosylation (SIRT4 and 6). [12] Discovery of diverse molecular targets are implicating that the SIRTs are important regulators of diverse physiology, including apoptosis and cell proliferation. Indeed, as shown in lower organisms, expression levels of SIRT1 in mammals are increased in response to CR. [13],[14] Among seven SIRTs, SIRT1 has been investigated most intensively, because the function of SIRT1 in cancer is complex and still not well understood. SIRT1 has been shown as both a promoter and a suppresser of tumor development. [15] Previous data suggest that SIRT1 function as an oncogene and play a role in carcinogenesis. [16],[17],[18] SIRT1 may inhibit apoptosis by deacetylating its targets that include tumor suppressor p53. [19] On the other hand, several studies have indicated that SIRT1 has tumor-suppressive functions. Wang et al. showed that resveratrol, that is activator of SIRT1, suppressed the expression of Survivin. [20] Survivin is one of the most cancer-specific genes, with essential roles in suppression of cell death, mitotic progression, and cellular adaptation. [21] SIRT1 may promote cancer-specific cell death by suppressing Survivin, an inhibitor of apoptosis that is highly expressed in most human tumors. [20] SIRT1 also prevents cancer by suppressing oncogenes, such as β-catenin. It has been shown that SIRT1 overexpression inhibits the growth of colon cancer cells dependent on β-catenin activity, suppresses the localization of β-catenin to the nucleus, and significantly attenuates its ability to activate transcription. [22]
The induction of colonic tumors in mice and rats by DMH is widely used as an experimental model for studies on the role of dietary factors in colon carcinogenesis. [23] Multiple injections of DMH result in neoplastic changes similar to those of human with regard to response to chemotherapy or chemopreventive agents. Aberrant crypt foci (ACF) are putative preneoplastic lesions of the colonic mucosa in mice, rats, and humans. [24] ACF with varying growth features are reported to occur in a rat colon after injections of a colon carcinogen, [25],[26] presumably representing preneoplastic lesions at different developmental stages. Studies have supported the concept that ACF with a higher crypt multiplicity are more likely to develop into neoplastic lesions. [27],[28]
In this study, to elucidate the molecular mechanisms underlying the protection of colon carcinogenesis by CR, we determine whether CR would modify the growth of preneoplastic ACF. We also analyzed the changes of SIRTs genes expression patterns by CR and compared to the ACF formation in DMH-treated rat colon mucosa.
Materials and Methods
Chemicals
DMH was purchased from Sigma-Aldrich (St. Louis, MO).
Animals
Four-week-old male Fisher 344 rats were purchased from Shizuoka Laboratory Animal Center (Hamamatsu, Japan). All rats were housed 3 or 4 per wire cage under controlled conditions of humidity (50 ± 10%), temperature (23 ± 2C), and kept in a 12/12 hour light-dark cycle. This experiment was performed according to the guidelines for the Animal Experimentation University of the Ryukyus and was approved by the Animal Care and Use Committee, University of the Ryukyus.
Treatment of rats
The experimental design is shown in [Figure 1]. A total of 56 rats were randomly divided into four experimental groups. Rats in groups 1 and 2 were given two weekly subcutaneous injection of DMH (40 mg/kg body weight), and those in groups 3 and 4 were injected with saline subcutaneously. Rats in groups 1 and 3 were fed a standard rodent diet (CE-2, CLEA, Tokyo, Japan) ad libitum, and those in groups 2 and 4 were fed a daily food allotment of 60% of that eaten by the ad libitum animals. Water was available ad libitum for both groups.
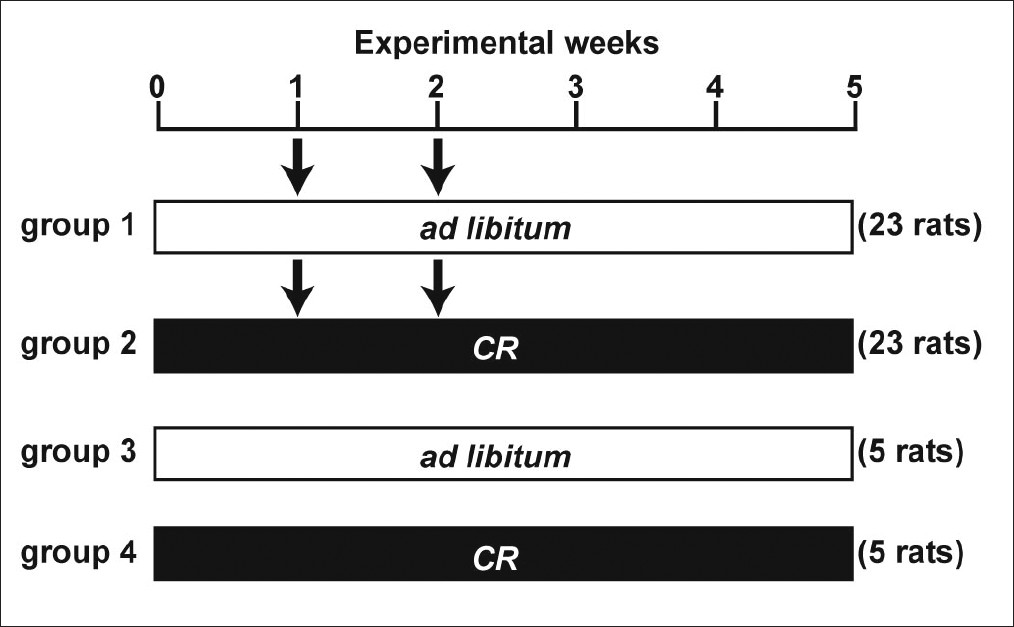 |
Figure 1: Experimental protocol. Arrow indicates subcutaneous injection of DMH (40 mg/kg body weight). CR, caloric restriction (60% of the ad libitum intake). Click here to view |
Determination of ACF
Animals were sacrificed at 5 weeks after the beginning of the experiment [Figure 1]. The entire colon was removed, gently flushed with saline to remove any fecal contents, opened longitudinally, and fixed in 10% neutralized formalin. Colon tissues were stained with 0.2% methylene blue solution for 30 s, immediately washed with distilled water, and then placed on a glass plate with the mucosal surface up. Using a stereomicroscope at a magnification of ×40, ACF were counted according to the criteria described earlier. [25]
Immunohistochemical staining and measurement for proliferating cell nuclear antigen labeling index
The proliferating cell nuclear antigen (PCNA) labeling index was evaluated to determine the proliferative activity of the colonic epithelial cells. PCNA assays were carried out on the colonic mucosae of 13 rats from each group (group 1 or group 2). The embedded tissues were cut into 4-μm sections and then stained using an anti-PCNA antibody (Dako, Carpinteria, CA,) and EnVision+ system-HRP Labeling Polymer (Dako). The anti-PCNA antibody was used at a dilution of 1:200. The number of PCNA-positive nuclei per section of the crypts was counted, and then percentages of PCNA-positive cells in each crypt were determined as the PCNA index.
Reverse transcriptase-PCR
The expressions of SIRT1-7, Survivin, and Cyclin D1 mRNA in the colonic mucosal cells were evaluated by reverse transcriptase-PCR (RT-PCR). Mucosal cells were scraped from the colon immediately after sacrifice and frozen in nitrogen liquid. Total RNA was extracted from the colonic epithelium using Trizol Reagent (Invitrogen, Carlsbad, CA), and reverse transcribed to obtain single-strand cDNA with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). PCR was carried out with a 7300 Real-Time PCR System (Applied Biosystems). The PCR primer pairs used in this study for SIRT1-7, Survivin, Cyclin D1, and GAPDH were as follow: SIRT1, sense 5′-GACGATGACAGAGCATCACACGCA-3′, antisense 5′-CGAGGATCGGTGCCAATCATGAGG-3′, SIRT2, sense 5′-AGCCACCCCACGACTGCTCA-3′, antisense 5′-ACAGTCACCCAGCCAGGCCA-3′, SIRT3, sense 5′-ACAAGGAGCTGCTTCTGCGGC-3′; antisense 5′-CTGCCATCACGTCAGCCCGT-3′, SIRT4, sense 5′-CGCGCTGTGGAGAGCTGCTG-3′; antisense 5′-CCCTAGGCGTGGCTTTGGGC-3′, SIRT5, sense 5′-GGGTCCCGGTGGCCGAGTTTA-3′; antisense 5′-TGTCAGTGCCCTGCATCTGTGC-3′, SIRT6, sense 5′-ACATGTGCGCTCGGGCTTCC-3′, antisense 5′-CTTGGCCACGGTGCAGAGCC-3′, SIRT7, sense 5′-CCAAGCCGTCGGCCCAAACT-3′, antisense 5′-TCGCCAGCACGGAGTGGAGT-3′, Survivin, sense 5′-AGGACCACCGGATCTACACCTTC-3′, antisense 5′-ATTCTCGGTAGGGCAGTGGATG-3′, Cyclin D1, sense 5′-GCTGCTGGGAGTGTGGTGGC-3′, antisense 5′-ATGTTCTGCTGGGCCTGGCG-3′, GAPDH, sense 5′-GACAACTTTGGCATCGTGGA-3′; antisense, 5′- ATGCAGGGATGATGTTCTGG-3′. All PCR reactions were run in triplicates and mRNA expression, relative to GAPDH, was calculated using the ΔΔCt method. [29]
Western blotting
Western blot analysis was performed as described previously. [30] In brief, whole tissue lysates were subjected to SDS-PAGE and electroblotted onto polyvinylidene difluoride membranes (Millipore, Billerica, MA), and then analyzed for immunoreactivity with the appropriate primary and secondary antibodies as indicated in the figures. Reaction products were visualized using enhanced chemiluminescence reagent, according to the instructions provided by the manufacturer (GE Healthcare, Waukesha, WI). Anti-Survivin (Cell Signaling Technology, Danvers, MA), anti-Cyclin D1 (MEDICAL & BIOLOGICAL LABORATORIES, Nagoya, Japan), and anti-actin (Lab Vision, Fremont, CA) antibodies were used. Horseradish-peroxidase-conjugated secondary antibodies were purchased from GE Healthcare.
Statistical analysis
All data were expressed as the mean ± SD. Differences in data between the animals of experimental groups were analyzed by Student’s or Welch’s t-test. P < 0.05 was considered statistically significant.
Results
Only CR decreased the body weight of rats
Rats in each group were monitored for food intake and body weight once a week [Figure 2]. CR rats were fed a daily food allotment of 60% of that eaten by the ad libitum animals. Food consumption of ad libitum rats in the experimental groups did not vary. As expected, body weights of animals fed the CR diets were lower than those fed the ad libitum diet in both DMH- and vehicle-treated groups throughout the study (*P < 0.05). DMH-injection did not affect food intake and body weight [Figure 2].
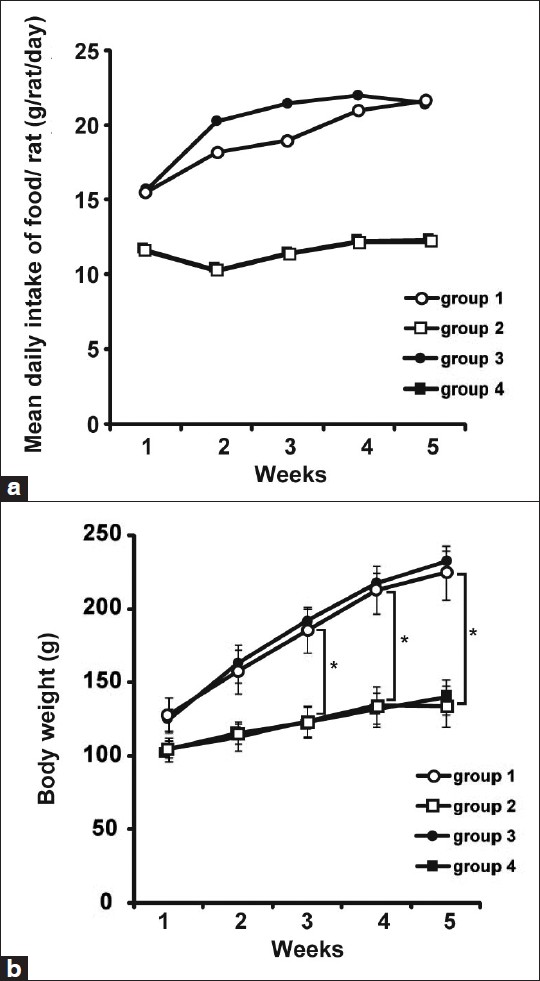 |
Figure 2: Food intake and body weight. (a) The mean daily intake of food for the different groups is shown. (b) Body weights of animals fed a calorie-restricted diet (groups 2 and 4) were lower than those fed ad libitum (groups 1 and 3) throughout the study (*P < 0.05). DMH treatment did not affect the body weight of rats Click here to view |
CR inhibited DMH-induced ACF formation
To examine the effect of CR on the promotion of colonic epithelial cell proliferation, the formation of chemically induced ACF was examined in colon specimens as a marker of early stage colorectal carcinogenesis. [26],[31] ACF were present throughout the length of the colon in the rats which were given with DMH-injection. The total number of ACF was significantly lower in CR rats (group 2) than that in ad libitum rats (group 1) (* P < 0.05) [Figure 3]a. The number of ACF containing one to three aberrant crypts was higher than that of more than four aberrant crypts in both groups. Both of them were also decreased significantly in CR rats compared to ad libitum rats (*P < 0.05) [Figure 3]b. No ACF was seen in any of the rats without DMH-injection (groups 3 and 4) (data not shown).
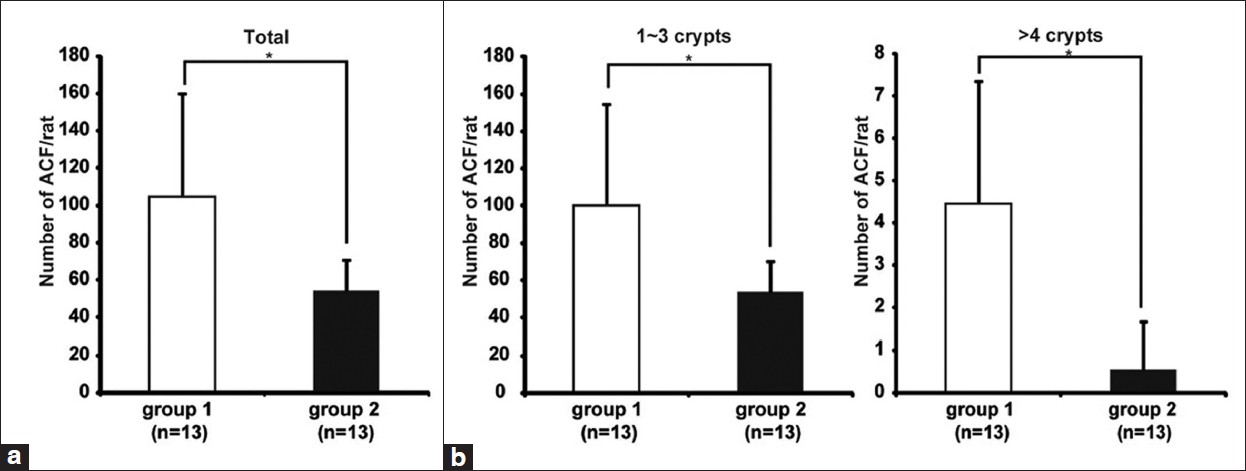 |
Figure 3: CR inhibited DMH-induced ACF formation. Colon tissues were stained in a 0.2% methylene blue solution. ACF were counted according to the Bird’s criteria.[25] (a) The average number of ACF induced by DMH-treatment was significantly lower in CR rats (group 2) than ad libitum rats (group 1) (*P < 0.05). (b) The average number of ACF containing one to three (left panel) or more than four (right panel) aberrant crypts was also decreased significantly in CR rats (*P < 0.05) compared with ad libitum rats Click here to view |
CR inhibited the DMH-induced PCNA labeling index in colonic mucosa
The proliferative activity of the colonic epithelial cells was determined by the PCNA labeling index [Figure 4]a. In group 2, the PCNA labeling index of the distal and middle regions and the entire colon was significantly lower than those of group 1. The numbers of the cells in a crypt of the distal and middle regions and the entire colon in group 2 were significantly lower than those in group 1 [Figure 4]b. Images of immunohistochemistory of PCNA are shown in [Figure 4]c. The length of each crypt in group 1 rats was longer than that in group 2 rats [[Figure 4]c, upper panels]. Moreover, PCNA-positive cells were increased particularly in the bottom of the crypt in group 1 rats [[Figure 4]c, lower panels]. These results indicated that the proliferative activity of the colonic epithelial cells was inhibited by CR, resulting in a decrease in the number of ACF.
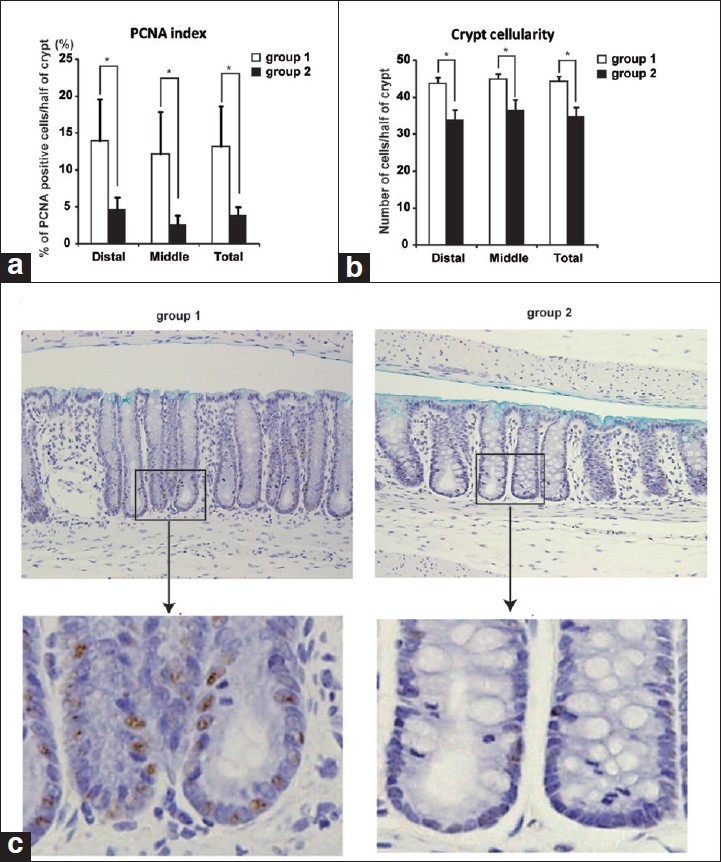 |
Figure 4: CR inhibited DMH-induced cell proliferation in colonic mucosa. Proliferating activity of the cells in colonic crypts was evaluated by measuring PCNA-positive nuclei. (a) Percentage of PCNA-positive nuclei of epithelial cell(PCNA index) in a half crypt was determined by immunohistochemistry. CR decreased the PCNA index significantly (*P < 0.05). (b) The number of cells of the crypt in distal and middle segments of the colon was counted. Data are mean ± SD. Number of the cells in a crypt was decreased significantly in CR rats (*P < 0.05). (c) Immunohistchemistry of PCNA in colonic mucosa of the rats in group 1 (left panels) and group 2 (right panels). Upper images are lower magnification (×20). Lower images are higher magnification of indicated upper images. Click here to view |
CR altered anti-apoptotic and cell cycle-related gene expressions
The levels of anti-apoptotic gene Survivin and cell cycle associated gene Cyclin D1 protein and mRNA transcript levels were determined in the colonic mucosae of rats in each group by Western blotting and real-time RT-PCR, respectively. Survivin protein levels in the colonic mucosae were reduced by CR in both DMH-treated and -untreated rats [Figure 5]a. Survivin mRNA levels were increased by DMH treatment (group 1 vs. group 3) and decreased by CR (group 1 vs. group 2). Those were lower in DMH-untreated CR rats compared to DMH-untreated ad libitum rats, but were not significant [Figure 5]b. Both Cyclin D1 protein [Figure 5]a and mRNA [Figure 5]b levels were also increased by DMH-treatment (group 1 vs. group 3) and returned to the same levels of DMH-untreated rats (group 2 vs. group 3).
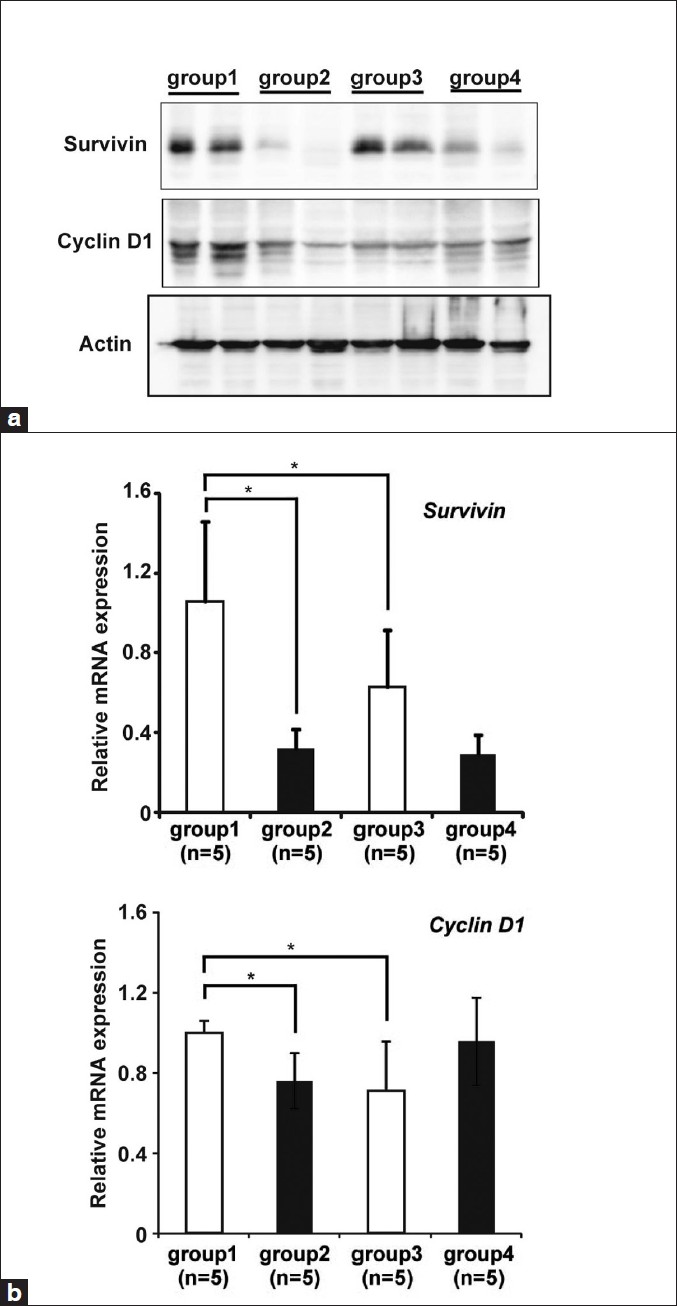 |
Figure 5: CR altered anti-apoptotic and cell cycle related gene expression. (a) The protein expressions of Survivin, anti-apoptotic protein and Cyclin D1, progression of cell cycle-related protein were analyzed by western blot. Two representative samples from each group are shown. Actin was a loading control. (b) The expression of Survivin and Cyclin D1 mRNA was analyzed by real-time RT-PCR. Results are shown as fold change of mRNA expression relative to that of mean value of group 1. Real-time RT-PCR data were obtained using the ΔΔ Ct method, with normalization to the reference GAPDH mRNA (mean ± SD, n=5, *P<0.05) Click here to view |
CR altered SIRTs gene expression in colonic mucosa
To clarify the mechanisms underlying the reduced proliferative activity of the colonic epithelial cells in the CR group with DMH-treatments, we investigated the expression levels of 7 SIRT family genes (SIRT1-7) in colon specimens in each group by real-time RT-PCR [Figure 6]. CR increased SIRT1-7 mRNA expression significantly in DMH-treated rats (group 1 vs. group 2), but not in DMH-untreated rats except SIRT1 (group 3 vs. group 4).
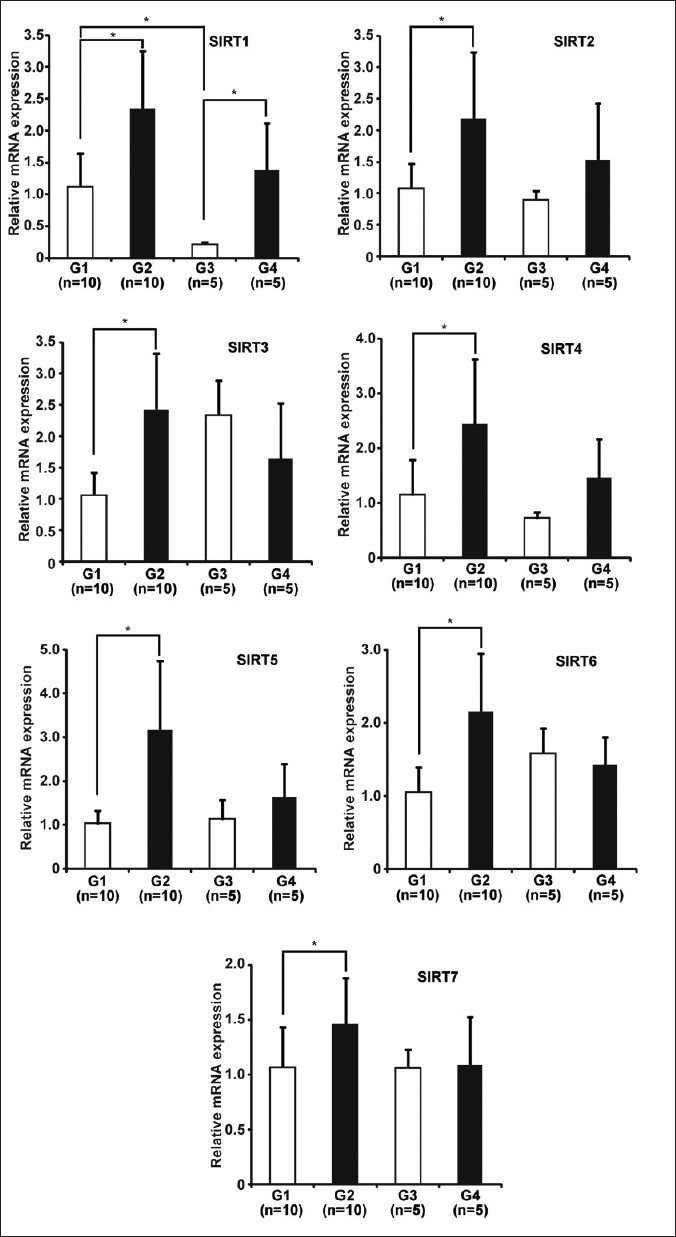 |
Figure 6: CR altered SIRTs gene expression in colonic mucosa of rats. Seven SIRT family genes (SIRT1-7) in colonic mucosal cells were analyzed by real-time RT-PCR. Results are shown as fold change of mRNA expression relative to that of mean value of group 1. Real-time RT-PCR data were obtained using the ΔΔ Ct method, with normalization to the reference GAPDH mRNA (mean ± SD, groups 1 and 2, n=10, groups 3 and 4, n=5, *P<0.05). Click here to view |
Discussion
Colorectal carcinogenesis is a multistep process that includes selection and propagation of preneoplastic lesions. Several studies have supported the contention that ACF are preneoplastic lesions in colon carcinogenesis by investigating the morphological and genotypic features of ACF. [24] In this study, we found that CR reduced the number of ACF formation in colonic mucosa in DMH-treated rats [Figure 3]. Total number of ACF-induced by DMH was significantly decreased by 5 weeks CR [Figure 3]a. The number of ACF with more than four aberrant crypts was also significantly decreased in CR rats [Figure 3]b. The concept, that ACF with a higher aberrant crypt multiplicity (number of crypts/focus) are more likely to develop into neoplastic lesions, [28] suggest that ACF with increasing crypt multiplicity exhibit increasing potential to develop into cancer. According to this theory, our results suggest that only a short-term (5 weeks) CR suppress not only the occurrence of preneoplastic lesions but also their development into neoplastic lesions in the colon of DMH-treated rats.
In this study, we used 60% food-restricted protocol for CR. Is that too strict restriction? Severity of CR is the age when CR is started and the strain of the animals determine the magnitude of cancer prevention. In general, an effect of CR seems to be modest and required a longer duration in order to exert measurable responses at the tissue level. Indeed, previous study has shown that the ability of CR to significantly alter the development of ACF was not evident at week 4 but significant alteration was seen only at week 12. [32] In contrast, we observed significant alteration of DMH-induced ACF formation in 5 weeks after starting CR diet. The condition of CR (60% of ad libitum diet) that we used in this study might be most strict condition comparing with previous papers. In fact, the average of body weights of CR rats was lower than ad libitum rats during the entire experiments. It was not dependent on DMH treatments [Figure 2]. However, mortality rate and physical activity of these rats were not different between CR and ad libitum rats (data not shown). These results indicate that our CR protocol was not too strict to make the animals malnutrition and too difficult to assess its effects on colon carcinogenesis.
How does CR decreases occurrence and development of precancerous lesions? What are the molecular mechanisms of that? Abnormal cell proliferation and inhibition of cell death are one of the important mechanisms in colon carcinogenesis. To understand the protective effects of CR against DMH-induced colon carcinogenesis, the expression of cell proliferation markers, such as PCNA and Cyclin D1, and anti-apoptotic protein, Survivin, were examined. PCNA is an auxiliary protein of the DNA polymerase δ, expressing in the nuclei of cells during the DNA synthesis, and playing an important role in cellular proliferation. In this study, rats treated with DMH alone showed intense nuclear staining for PCNA. PCNA-positive cells in those rats were seen at the bottom of the crypts, where intestinal epithelial cell renewals mainly occurre [[Figure 4]c, left panels]. Numbers of PCNA-positive cells were decreased by CR in DMH-treated rats [[Figure 4]c, right panels]. Moreover, CR decreased the expressions of anti-apoptotic gene Survivin and cell-cycle associated gene Cyclin D1 in DMH-treated rats both at mRNA [Figure 5]b and protein [Figure 5]a levels. Interestingly, CR itself did not change the mRNA expressions of these genes in DMH-untreated rats [Figure 5]b. These results suggest that CR prevents the initial stage of colon carcinogenesis by inhibiting excessive cell proliferation and induction of apoptosis of colonic mucosal cells.
Because CR extends the lifespan of many lower organisms, the nutrient-response pathways that regulate age-dependent phenotypes in these organisms can provide insights on the mechanisms linking CR and cancer reduction. Previous studies in lower organisms indicate that insulin-like growth factor 1 (IGF-I) pathways might be important for aging and cancer in mammals. [33] Downstream of IGF-I signaling pathways, PI3K/Akt signaling plays an important role for cell proliferation and inhibition of apoptosis. Previous studies suggested that several downstream effectors of PI3K/Akt, including SIRT1 might be responsible for anti-carcinogenic effects of CR. [22] Here, we analyzed the expression of all human SIRTs (SIRT1-7) in response to CR and/or DMH. We found that CR increased SIRT1-7 expression in colonic mucosal cells in DMH-treated rats [Figure 6]. Among seven mammalian SIRTs proteins, SIRT1 has been extensively studied. SIRT1 may promote cancer-specific cell death by suppressing Survivin. Moreover, SIRT1 prevents cancer by suppressing oncogenes, such as β-catenin. The accumulation of β-catenin in nucleus activates transcription of cyclin D1 gene in colon cancer cells. [34] Therefore, down-regulation of Cyclin D1 expression by CR in DMH-treated rats might be through suppression of β-catenin by SIRT1. As our DMH-induced colon cancer model animals, SIRT1-activation during CR could prevent carcinogenesis by increasing promoting cancer-specific cell death and suppressing cell proliferation. However, the roles of other SIRTs in human carcinogenesis are still unclear.
Conclusions
In conclusion, we demonstrated that CR reduced ACF formation and cell proliferation in colonic mucosal cells in DMH-treated rats. CR decreased anti-apoptotic protein Survivin and cell cycle associated protein Cyclin D1 in these cells. These results suggest that CR prevents the initial stage of colon carcinogenesis by inhibiting excessive cell proliferation and induction of apoptosis of colonic mucosal cells. CR also increased SIRT1-7 expression in colonic mucosal cells in DMH-treated rats. Previous studies have shown that SIRT1 prevents intestinal tumor formation. [22] Therefore, SIRT1 might have an important role in prevention ACF formation induced by DMH. However, the effects of other SIRTs (SIRT2-7) on the reduced ACF formation in CR rats were still unclear. Further molecular biological investigations will be needed to fully elucidate the roles of SIRTs in prevention of ACF formation and colon carcinogenesis.
References
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58:71-96.  [PUBMED] |
| 2. | Willett WC, MacMahon B. Diet and cancer–an overview. N Engl J Med 1984;310:633-8.  [PUBMED] |
| 3. | Byers T, Graham S. The epidemiology of diet and cancer. Adv Cancer Res 1984;41:1-69.  [PUBMED] |
| 4. | Reddy BS. Dietary fat and cancer: specific action or caloric effect. J Nutr 1986;116:1132-5.  [PUBMED] |
| 5. | Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev 2005;126:913-22.  [PUBMED] |
| 6. | Lasko CM, Good CK, Adam J, Bird RP. Energy restriction modulates the development of advanced preneoplastic lesions depending on the level of fat in the diet. Nutr Cancer 1999;33:69-75.  [PUBMED] |
| 7. | Klurfeld DM, Weber MM, Kritchevsky D. Inhibition of chemically induced mammary and colon tumor promotion by caloric restriction in rats fed increased dietary fat. Cancer Res 1987;47:2759-62.  [PUBMED] |
| 8. | Reddy BS, Wang CX, Maruyama H. Effect of restricted caloric intake on azoxymethane-induced colon tumor incidence in male F344 rats. Cancer Res 1987;47:1226-8.  [PUBMED] |
| 9. | Kumar SP, Roy SJ, Tokumo K, Reddy BS. Effect of different levels of calorie restriction on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res 1990;50:5761-6.  [PUBMED] |
| 10. | Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 2000;273:793-8.  [PUBMED] |
| 11. | Anderson RM, Latorre-Esteves M, Neves AR, Lavu S, Medvedik O, Taylor C, et al. Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science 2003;302:2124-6.  [PUBMED] |
| 12. | Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev 2006;20:2913-21.  [PUBMED] |
| 13. | Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004;305:390-2.  [PUBMED] |
| 14. | Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 2004;306:2105-8.  [PUBMED] |
| 15. | Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer 2009;9:123-8.  [PUBMED] |
| 16. | Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A, et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res 2007;67:6612-8.  [PUBMED] |
| 17. | Hida Y, Kubo Y, Murao K, Arase S. Strong expression of a longevity-related protein, SIRT1, in Bowen’s disease. Arch Dermatol Res 2007;299:103-6.  [PUBMED] |
| 18. | Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004;23:2369-80.  [PUBMED] |
| 19. | Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001;107:149-59.  [PUBMED] |
| 20. | Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell 2008;32:11-20.  [PUBMED] |
| 21. | Altieri DC. New wirings in the survivin networks. Oncogene 2008;27:6276-84.  [PUBMED] |
| 22. | Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One 2008;3:e2020.  [PUBMED] |
| 23. | Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog 2009;8:5.  [PUBMED]  |
| 24. | Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett 1995;93:55-71.  [PUBMED] |
| 25. | Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett 1987;37:147-51.  [PUBMED] |
| 26. | McLellan EA, Bird RP. Aberrant crypts: potential preneoplastic lesions in the murine colon. Cancer Res 1988;48:6187-92.  [PUBMED] |
| 27. | Zhang XM, Stamp D, Minkin S, Medline A, Corpet DE, Bruce WR, et al. Promotion of aberrant crypt foci and cancer in rat colon by thermolyzed protein. J Natl Cancer Inst 1992;84:1026-30.  [PUBMED] |
| 28. | Magnuson BA, Carr I, Bird RP. Ability of aberrant crypt foci characteristics to predict colonic tumor incidence in rats fed cholic acid. Cancer Res 1993;53:4499-504.  [PUBMED] |
| 29. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8.  [PUBMED] |
| 30. | Tomita M, Choe J, Tsukazaki T, Mori N. The Kaposi’s sarcoma-associated herpesvirus K-bZIP protein represses transforming growth factor beta signaling through interaction with CREB-binding protein. Oncogene 2004;23:8272-81.  [PUBMED] |
| 31. | Konstantakos AK, Siu IM, Pretlow TG, Stellato TA, Pretlow TP. Human aberrant crypt foci with carcinoma in situ from a patient with sporadic colon cancer. Gastroenterology 1996;111:772-7.  [PUBMED] |
| 32. | Lasko CM, Bird RP. Modulation of aberrant crypt foci by dietary fat and caloric restriction: the effects of delayed intervention. Cancer Epidemiol Biomarkers Prev 1995;4:49-55.  [PUBMED] |
| 33. | Longo VD. Linking sirtuins, IGF-I signaling, and starvation. Exp Gerontol 2009;44:70-4.  [PUBMED] |
| 34. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999;398:422-6.  [PUBMED] |
Authors
Dr. Mariko Tomita, Department of Pathology and Oncology, Graduate School of Medical Science, Universtiy of the Ryukyus, Nishihara, Okinawa, 903-0215, Japan
Figures
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6]
