Mohammad-Hassan Khadem-Ansari1, Zahra Shahsavari1, Yousef Rasmi1, Rahim Mahmoodlo2
1 Department of Biochemistry, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
2 Department of Surgery, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
| Date of Submission | 30-Jul-2010 |
| Date of Acceptance | 14-Jan-2011 |
| Date of Web Publication | 16-Apr-2011 |
Correspondence Address:
Mohammad-Hassan Khadem-Ansari
Department of Biochemistry, Faculty of Medicine, Urmia University of Medical Sciences, Urmia
Iran
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1477-3163.79683
Abstract
Aims:To measure oxidative DNA and lipid damages, urinary levels of 8-hydroxy-2Ͳ-deoxyguanosine (8-OHdG), and 8-isoprostane in esophageal squamous cell carcinoma (SCC) patients and compare the values with that in controls. Materials and Methods: The urinary concentrations of 8-OHdG and 8-isoprostane were measured in 32 SCC patients (13 female/19 male; mean age: 61.4 ± 10.5 years) and 45 controls (22 female/23 male; mean age: 58.1 ± 8.3 years). Results: Squamous cell carcinoma patients showed significantly higher levels of urinary 8-OHdG (15.6 ± 5.1 ng/mg creatinine) than controls (5.8 ± 2.1 ng/mg creatinine) (P<.001). Increased urinary concentrations of 8-isoprostane were also detected in SCC patients (35.4 ± 6.5 ng/mmol creatinine) as compared to the controls (16.9 ± 4.0 ng/mmol creatinine) (P<.001). Conclusions: Our results show the presence of oxidative DNA and lipid damage in the SCC patients. This may have a connection to carcinogenesis in the esophagus.
Keywords: Esophageal squamous cell carcinoma, 8-hydroxy-2′-deoxyguanosine, 8-isoprostane
How to cite this article:
Khadem-Ansari MH, Shahsavari Z, Rasmi Y, Mahmoodlo R. Elevated levels of urinary 8-hydroxy-2′-deoxyguanosine and 8-isoprostane in esophageal squamous cell carcinoma. J Carcinog 2011;10:14
How to cite this URL:
Khadem-Ansari MH, Shahsavari Z, Rasmi Y, Mahmoodlo R. Elevated levels of urinary 8-hydroxy-2′-deoxyguanosine and 8-isoprostane in esophageal squamous cell carcinoma. J Carcinog [serial online] 2011 [cited 2021 Oct 15];10:14. Available from: https://carcinogenesis.com/text.asp?2011/10/1/14/79683
Background
Esophageal cancer in humans occurs worldwide with a variable geographic distribution and ranks eighth among various cancers in the order of occurrence. This malignancy exists in two main forms, with distinct etiological and pathological characteristics: squamous cell carcinoma (SCC) and adenocarcinoma. More than 90% of cancers of the esophagus worldwide are SCCs. [1] SCC remains prevalent worldwide, particularly in the endemic areas of China, Russia, Turkey, and northern Iran. [2] Despite widespread efforts at developing methods for the early diagnosis of SCC over the past two decades there has been limited success. [3]
All cells in the body are exposed constantly to oxidants from both endogenous and exogenous sources and to free radicals that are continuously produced in vivo. Reactive oxygen and nitrogen species can attack various substrates in the body, such as lipids and nucleic acids. Oxidation of any of these substrates can theoretically contribute to chronic diseases like cancer. [4]
The most representative product that may reflect oxidative damage induced by reactive oxygen species (ROS) is 8-hydroxy-2′-deoxyguanosine (8-OHdG), a product of oxidatively modified DNA base guanine. [5] Urinary 8-OHdG has been reported to be strongly association with diabetes mellitus, [6] chronic renal failure, [7] and cancer. [8]
F2-Isoprostanes, a group of bioactive prostaglandin F2-like compounds generated by the oxidatively catalyzed reaction of arachidonic acid, are considered as reliable markers of lipid peroxidation in vivo. The 8-isoprostane (8-isoprostaglandin F2a, the major F2-isoprostane), the well-known compound belonging to the F2-isoprostane class is, in practice, usually quantified in urine instead of plasma because of the short half-life of plasma F2-isoprostane. Elevated levels of urinary 8-isoprostane has been reported in several conditions, such as diabetes, alcoholic liver disease, cardiovascular disease, and cancer. [9]
In the present study, we measured oxidative DNA and lipid damage by determining the levels of 8-OHdG and 8-isoprostane in SCC as compared to controls.
Subjects and Methods
Thirty-two patients with SCC proven by biopsy and pathological examination (19 male/13 female; mean age: 61.4 ± 10.5), referred to our hospital for treatment, were selected for the study. Forty-five volunteers (23 male/22 female; mean age: 58.1 ± 8.3), hospitalized for non-kidney diseases, were used as controls. Written informed consent was obtained from patients and control participants. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the guidelines of the Medical Ethics Committee, Ministry of Health, Iran. Hospital records were used to verify patients’ data. Subjects who had any history of stroke, diabetes, ischemic heart disease, asthma, or kidney disease and those who were taking any kind of medicines or supplements such as vitamins were excluded. Twenty-four hoursurine samples from patients with SCC were collected before surgery. Similarly, 24-hours urine samples collections were obtained from controls. All the samples were centrifuged at 10000 g for 10 min to remove any precipitate. The supernatants were stored at −40°C until analyses. A competitive ELISA kit (Cayman Chemicals, Ann Arbor, Michigan, USA) was used for determination of 8-OHdG in the urine samples. Determination of 8-OHdG level was conducted according to the kit protocol, using the mouse anti-mouse IgG-coated plate provided in the kit and a standard curve of 8-OHdG. The urinary concentration of 8-OHdG was expressed by creatinine to avoid the effect of urine volume fluctuation [8-OHdG (ng/ml): creatinine (mg/ml)]= 8-OHdG (ng/ mg creatinine). Similarly, a competitive ELISA kit (Cayman Chemicals, Ann Arbor, Michigan, USA) was used to detect the levels of 8-isoprostane produced within the esophageal cancer patients and the controls. Determination of 8-isoprostane levels in each sample was conducted according to the kit protocol, using the mouse anti-rabbit IgG coated plate provided in the kit and a standard curve of 8-isoprostane. The urinary concentration of 8-isoprostane was expressed by creatinine to avoid the effect of urine volume fluctuation [8-isoprostane (ng/ml): creatinine (mmol/ml)]= 8-isoprostane (ng/mmol creatinine). Statistical analysis was performed using SPSS software (version 16). Statistical comparison of the results was performed using an independent samples t-test. Data were expressed as mean ± SD for each group. Statistical significance was determined at P<.05.
Results
Demographic and clinical characteristics of controls and SCC patients are shown in [Table 1].
| Table 1: Demographic and clinical characteristics of controls and SCC patients Click here to view |
SCC patients had significantly elevated urine levels of 8-isoprostane compared with controls (35.4 ± 6.5 vs 16.9 ± 4.0 ng/mmol creatinine, respectively; P=.000) [Figure 1].
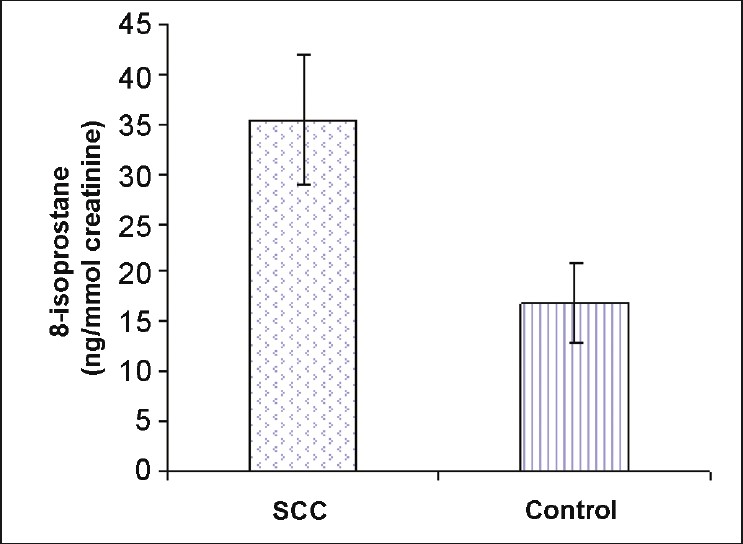 |
Figure 1: Elevated levels of urinary 8-isoprostane (ng/mmol creatinine) in SCC patients as compared with controls (P<.001). Values are expressed as mean ± SD. Click here to view |
As shown in [Figure 2], increases in 8-OHdG were found in patients with SCC (15.6 ± 5.1 ng/mg creatinine). The mean values obtained in SCC patients were significantly different when compared with that of controls (5.8 ± 2.1 ng/mg creatinine) (P=.000) [Figure 2].
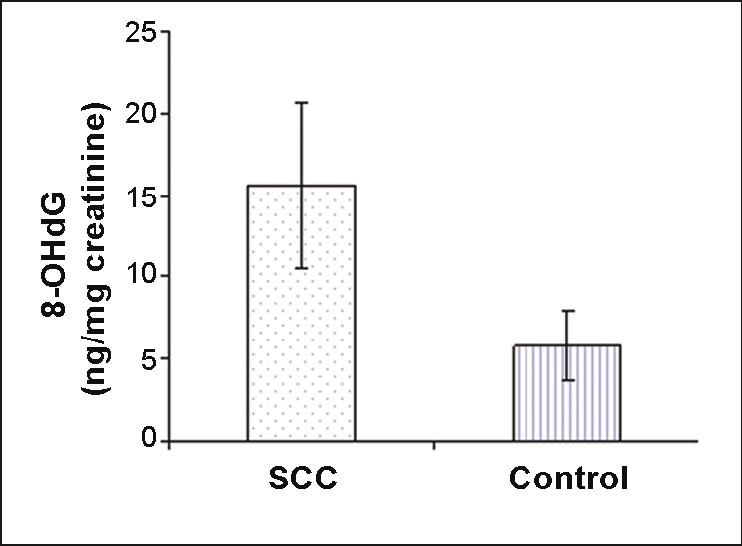 |
Figure 2: Levels of urinary 8-OHdG (ng/mg creatinine) in SCC patients and controls (P<.001). Values are expressed as mean ± SD. Click here to view |
Discussion
The incidence of esophageal cancer varies widely among different geographic regions. The high risk areas include the so-called Asian esophageal cancer belt from eastern Turkey, through the southern regions of the former Soviet union (Kazakhstan, Turkmenistan, Uzbekistan, and Tajikistan), Iraq, Iran into western and northern China, Hong Kong, Japan, southeastern Africa, France, and parts of South America (Brazil and Bermuda). It has been reported that in certain regions of northern China or the Caspian Sea littoral in Iran, SCC is 200 times more likely to develop than in other low-risk areas of the world. [3] The etiology of SCC, particularly in people living in high-risk regions such as the Caspian Sea littoral region of Iran, is unclear. [10]
Numerous epidemiological studies and intervention studies with antioxidants indicate that oxidative modification is an important factor in cancer development at certain sites.[11] Oxidative stress is well documented as a biological phenomenon. It has been demonstrated that oxidative stress leads to oxidation of important macromolecules, and this has been hypothesized to be an important pathogenic factor in the development of cancer. [12] The present study investigated the association of oxidative stress, as assessed by 8-OHdG and 8-isoprostane, with carcinogenesis in the esophagus. The substances 8-OHdG and 8-isoprostane are known to indicate oxidative damage of DNA and membrane lipid, respectively. There have been no studies that have evaluated the association of this oxidative stress-related 8-OHdG and 8-isoprostane in the urine of SCC patients.
Estimation of 8-OHdG is an important factor in the evaluation of oxidative DNA damage. It has been shown that hydroxyl radicals, singlet oxygen, and direct photodynamic action produce 8-hydroxylation of the guanine base. Damaged DNA is repaired in vivo by endonucleases and free water-soluble 8-OHdG is excreted into the urine without further metabolism. [13] We observed that the urinary 8-OHdG level was significantly higher in SCC patients compared with controls.
In 1995 Toyokuni et al. reported that human carcinoma cells (breast, lung, liver, kidney, brain, stomach, and ovary) have a higher content of 8-OHdG than adjacent non-tumorous tissue. [14] Moreover, investigators have reported a high concentration of 8-OHdG in renal cell carcinoma cells, [15] as well as in urine samples from patients with carcinoma of female genitalia, [16] in malignant breast tissues with invasive ductal carcinoma, [17] and in colorectal tumor tissues, [18] gastric cancer tissues, [19] and lung cancer tissues. [10] They hypothesized that the tumor cells themselves produce ROS spontaneously, which results in an increase of 8-OHdG in DNA. Tagesson et al. in 1995 reported significant elevation of urinary 8-OHdG excretion following chemotherapy in various cancer patients (i.e., patients with laryngeal cancer, osteosarcoma, and gastric cancer). [20] Erhola et al. in 1997 reported the elevation of urinary 8-OHdG in lung cancer patients, which decreased to pre-therapy levels after treatment. In contrast, no significant increase in urinary 8-OHdG was observed in patients whose disease were in progression. [21] It appears that urinary 8-OHdG is useful for indicating cancer risk. [11] Although the cause of increased urinary 8-OHdG levels in cancer patients is still unclear, this marker may be a good prognostic indicator. [13]
It has since become clear that measurement of F2-isoprostanes provides a valuable and reliable approach for assessing oxidative stress status in vivo. Measurement of F2-isoprostanes has firmly established the occurrence of oxidative stress in a wide variety of disease states, often for the first time. [22] Evidence is increasing that isoprostanes, a novel class of prostaglandin-like compounds produced upon peroxidation of lipoproteins, play a causative role in carcinogenesis. In human controls, levels of the 8-isoprostane range from 5-50 pg/ml plasma and 500-3000 pg/g urinary creatinine, respectively. The in vivo concentration of F2-isoprostanes increases dramatically in animal models of lipid peroxidation. Urine is generally considered a better matrix than serum to quantify isoprostane status. [23]
An increase in 8- isoprostane was not detected in the urine of patients with prostate cancer compared with normal controls. [24] In our study, there was a significant increase of urinary 8-isoprostane levels in SCC patients as compared with controls.
Conclusions
The results indicate that elevated levels of 8-OHdG and 8-isoprostane are seen in SCC patients, supporting the hypothesis that the evaluation of oxidative stress may represent an additional information. This may indicate an association between oxidative DNA and lipid damage and carcinogenesis of esophagus, and more studies are needed to clarify the picture.
References
| 1. | Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis 2001;22:1737-46.  [PUBMED] [FULLTEXT] |
| 2. | Khushalani N. Cancer of the esophagus and stomach. Mayo Clin Proc 2008;83:712-22.  [PUBMED] [FULLTEXT] |
| 3. | Mohammadzadeh GS, Nasseri Moghadam S, Rasaee MJ, Zaree AB, Mahmoodzadeh H, Allameh A. Measurement of glutathione S-transferase and its class-pi in plasma and tissue biopsies obtained after laparoscopy and endoscopy from subjects with esophagus and gastric cancer. Clin Biochem 2003;36:283-8.  [PUBMED] [FULLTEXT] |
| 4. | Hwang ES, Kim GH. Biomarkers for oxidative stress status of DNA, lipids, and proteins in vitro and in vivo cancer research. Toxicology 2007;229:1-10.  [PUBMED] [FULLTEXT] |
| 5. | Sakano N, Wang DH, Takahashi N, Wang B, Sauriasari R, Kanbara S, et al. Oxidative stress biomarkers and lifestyles in Japanese healthy people. J Clin Biochem Nutr 2009;44:185-95.  [PUBMED] [FULLTEXT] |
| 6. | Kanauchi M, Nishioka H, Hashimoto T. Oxidative DNA damage and tubulointerstitial injury in diabetic nephropathy. Nephron 2002;91:327-9.  [PUBMED] [FULLTEXT] |
| 7. | Akagi S, Nagake Y, Kasahara J, Sarai A, Kihara T, Morimoto H, et al. Significance of 8-hydroxy-2′-deoxyguanosine levels in patients with chronic renal failure. Nephrology (Carlton) 2003;8:192-5.  [PUBMED] [FULLTEXT] |
| 8. | Chiou CC, Chang PY, Chan EC, Wu TL, Tsao KC, Wu JT. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clin Chim Acta 2003;334:87-94.  [PUBMED] [FULLTEXT] |
| 9. | Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett’s esophagus patients. Nutr Cancer 2006;54:148-56.  [PUBMED] |
| 10. | Sharifi R, Allameh A, Biramijamal F, Mohammadzadeh SH, Rasmi Y, Tavangar SM, et al. Relationship between genetic polymorphism of glutathione S-transferase-p1 and p53 protein accumulation in Iranian esophageal squamous cell carcinoma patients. Indian J Cancer 2008;45:8-12.  [PUBMED] |
| 11. | Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta 2004;339:1-9.  [PUBMED] [FULLTEXT] |
| 12. | Poulsen HE. Oxidative DNA modifications. Exp Toxicol Pathol 2005;57:161-9.  |
| 13. | Honda M, Yamada Y, Tomonaga M, Ichinose H, Kamihira S. Correlation of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), a biomarker of oxidative DNA damage, and clinical features of hematological disorders: a pilot study. Leuk Res 2000;24:461-8.  [PUBMED] [FULLTEXT] |
| 14. | Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett 1995;358:1-3.  [PUBMED] [FULLTEXT] |
| 15. | Okamoto K, Toyokuni S, Uchida K, Ogawa O, Takenewa J, Kakehi Y, et al. Formation of 8-hydroxy-2′-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer 1994;58:825-9.  [PUBMED] |
| 16. | Yamamoto T, Hosokawa K, Tamura T, Kanno H, Urabe M, Honjo H. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels in women with or without gynecologic cancer. J Obstet Gynaecol Res 1996;22:359-63.  [PUBMED] |
| 17. | Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer 1996;32:1209-14.  |
| 18. | Oliva MR, Ripoll F, Muٌiz P, Iradi A, Trullenque R, Valls V, et al. Genetic alterations and oxidative metabolism in sporadic colorectal tumors from a Spanish community. Mol Carcinog 1997;18:232-43.  |
| 19. | Lee BM, Jang JJ, Kim HS. Benzo[a]pyrene diol-epoxide-I-DNA and oxidative DNA adducts associated with gastric adenocarcinoma. Cancer Lett 1998;125:61-8.  [PUBMED] [FULLTEXT] |
| 20. | Tagesson C, Källberg M, Klintenberg C, Starkhammar H. In vivo Determination of urinary 8-hydroxydeoxyguanosine by automated coupled-column high performance liquid chromatography: a powerful technique for assaying oxidative DNA damage in cancer patients. Eur J Cancer 1995;31:934-40.  |
| 21. | Erhola M, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, et al. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2′-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett 1997;409:287-91.  [PUBMED] [FULLTEXT] |
| 22. | Montuschi P, Barnes PJ, Roberts LJ 2nd. Isoprostanes: markers and mediators of oxidative stress. FASEB J 2004;18:1791-800.  [PUBMED] [FULLTEXT] |
| 23. | Thompson HJ, Heimendinger J, Gillette C, Sedlacek SM, Haegele A, O’neill C, et al. In vivo investigation of changes in biomarkers of oxidative stress induced by plant food rich diets. J Agric Food Chem 2005;53:6126-32.  [PUBMED] [FULLTEXT] |
| 24. | Camphausen K, Ménard C, Sproull M, Goley E, Basu S, Coleman CN. Isoprostane levels in the urine of patients with prostate cancer receiving radiotherapy are not elevated. Int J Radiat Oncol Biol Phys 2004;58:1536-9.  |
Figures
[Figure 1], [Figure 2]
Tables
